Dissolution of Ammonium Nitrate in Water Equation
What is the pH of this solution at equilibrium. Ammonium nitrate dissolving in solution is an endothermic reaction.

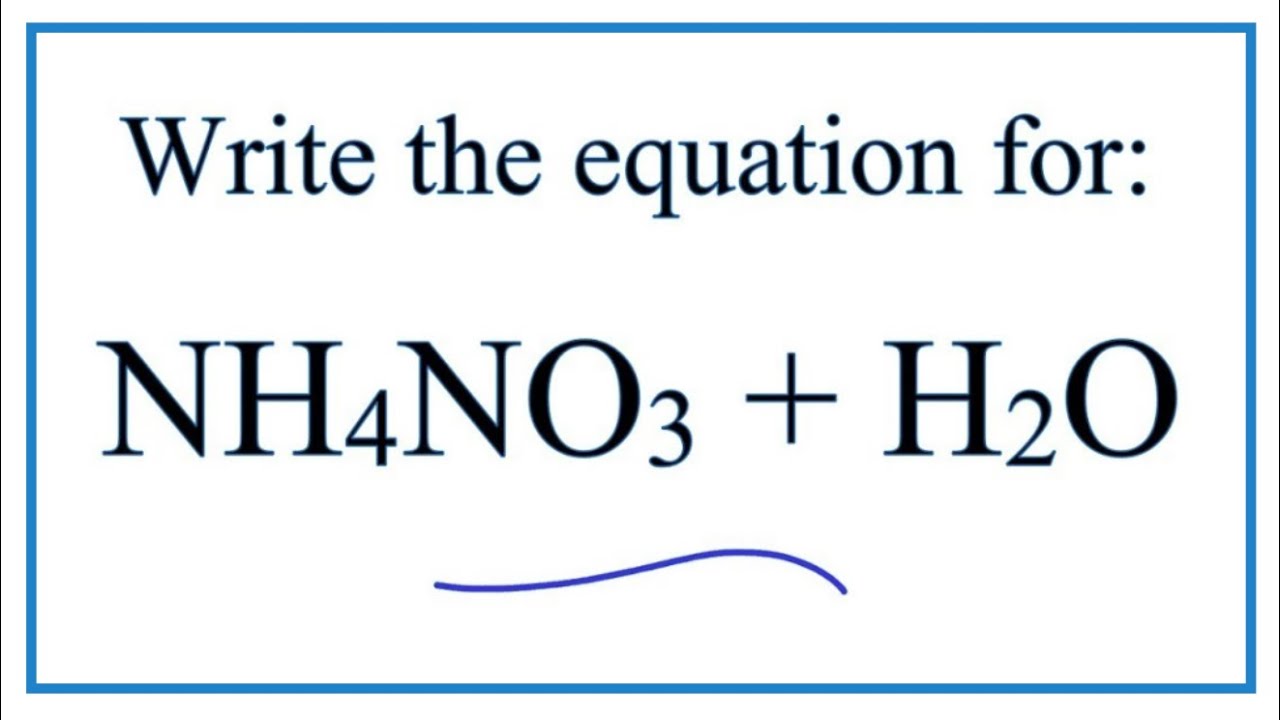
Equation For Ammonium Nitrate Dissolving In Water Nh4no3 H2o Youtube
Include states of matter.
. Write the balanced complete ionic equation for the following. Enter noreaction if no precipitate is formed. Is ammonium nitrate dissolving in water exothermic.
Write the formula for the dissolution of aluminum perchlorate in water. The dissolution of ammonium nitrate in water is given by the following equation. What Is Ammonium Nitrate Nh4no3 Facts Structure Properties Uses.
NH4NO3 3040 kJmol. 125mL Erlenmeyer flask w 30mL of water 3g NH 4 NO 3. How many TOTAL ions aluminum cations perchlorate anions would be produced as aqueous species in the water solvent if.
NH 4 NO 3 s NH 4 aq NO 3 aq TOPICS COVERED. MgBr2s H20 -- Mg2 aq 2Br- aq. The reactants and products would appear at a low energy state.
Chemistry questions and answers. Write a balanced chemical equation for the dissolution of solid ammonium nitrate in water. The equilibrium constant Keq is 18 105.
Here is the chemical equation for dissolving write net ionic solid ammonium nitrate chegg an 6 balanced and water reaction between nh4no3 h2o uses formula of saccharides. When ammonium nitrate is dissolved in water it feels cold which indicates an endothermic reaction. Endothermic reactions dissolution solubility energy transformations.
Often used to ice athletic injuries on the field contain ammonium nitrate and water separated by a thin plastic divider. Consider the dissociation of ammonia in water at equilibrium. As the ammonium nitrate dissolves heat energy is absorbed from the environment causing the surrounding environment to feel cold.
Identify all of the phases in your answer. NaIaqHg2NO32aq Express your answer as a chemical equation. NH4NO3s heat NH4aq NO3-aq Which statement best describes what an energy graph of this reaction would look like.
Write the balanced chemical equation for. NO3- 1009 kJmol. Write the equation for the dissolution of magnesium bromide in water.
The dissolution of ammonium nitrate in water is given by the following equationNH4NO3s heat NH4aq NO3-aq. 2H2O N2O This is possible but what you asked for. Ammonium nitrate dissolves in water via the following reaction.
NH4NO3 s NH4 aq NO3- aq The bond energies of the compounds in the reaction are as follows. NH4 1564 kJmol. The reactants would appear at a lower energy state than the products.
Write the equation for the dissolution of potassium iodide in water. OF POTASSIUM NITRATE DISSOLVING IN WATER VERSION V OBJECTIVE The ΔG ΔH and ΔS of the potassium nitrate KNO3 dissolving saturated potassium nitrate KNO3 solution in water H2O a dynamic equilibrium will be established and the reaction equation is shown in equation 1. The dissolution of ammonium nitrate in water is given by the following equation.
What is the chemical equation for ammonium nitrate decomposes into nitrogen and water. The correct chemical equation that represents the dissolution of ammonium nitrate in water istexbold NH_4NO_3s rightarrow NH4aq NO3-aqtexHo. Note that although liquid water is important to the dissolution its participation is implied in the states of matter.
You do NOT need to include H20 1 in your equation. Add NH 4 NO 3. KIs H20 -- K aq I- aq 2.
Balanced symbol equation for ammonium nitrate and water reaction between nh4no3 h2o dissolving an uses formula 6 undergoes frozen board endothermic difference quality standards total ammonia. NH 4 NO 3 can be prepared from the acid-base reaction between nitric acid and ammonia described by the following chemical equation. NH3 H2O NH4 OH You start with 005 moles of ammonia in 500 mL of water.
Ammonium nitrate readily dissolves in water by dissociating into its constituent ions. Is ammonium nitrate dissolving in water exothermic or endothermic. Ammonium nitrate dissolving in solution is an endothermic reaction.
As the ammonium nitrate dissolves heat energy is absorbed from the environment causing the surrounding environment to feel cold. As a result dissolving ammonium chloride in water will be an endothermic process. That contains 100 g of crystalline ammonium nitrate within the calorimeter.
This salt is acidic in nature since it is derived from a weak base NH3 and a strong acid HNO 3. NH4NO3s heat NH4aq NO3-aq.

Equation For Ammonium Nitrate Dissolving In Water Nh4no3 H2o Youtube

Solved 9 18 Points Here Is The Chemical Equation For The Chegg Com
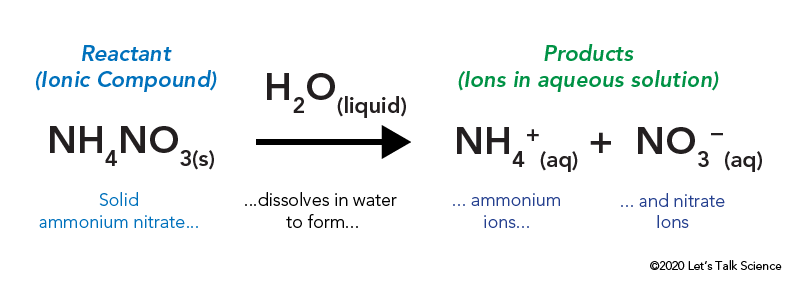
The Cold Pack A Chilly Example Of An Endothermic Reaction Let S Talk Science
Comments
Post a Comment